Easy Way to Know Polar or Nonpolar
4.12: Shapes and Properties- Polar and Nonpolar Molecules
- Page ID
- 351234
- Determine if a molecule is polar or nonpolar.
Molecular Polarity
To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons. Notice that a tetrahedral molecule such as \(\ce{CCl_4}\) is nonpolar Figure (\(\PageIndex{1}\). Another non polar molecule shown below is boron trifluoride, BF3. BF3 is a trigonal planar molecule and all three peripheral atoms are the same.

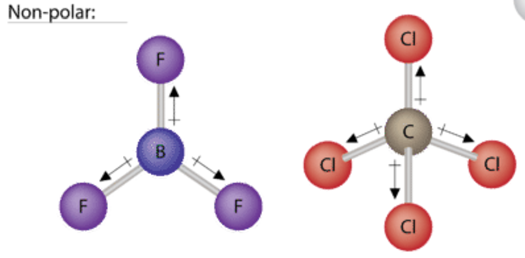

Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms with different electronegativities bonded. This works pretty well - as long as you can visualize the molecular geometry. That's the hard part. To know how the bonds are oriented in space, you have to have a strong grasp of Lewis structures and VSEPR theory. Assuming you do, you can look at the structure of each one and decide if it is polar or not - whether or not you know the individual atom electronegativity. This is because you know that all bonds between dissimilar elements are polar, and in these particular examples, it doesn't matter which direction the dipole moment vectors are pointing (out or in).
A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. A diatomic molecule that consists of a polar covalent bond, such as \(\ce{HF}\), is a polar molecule.
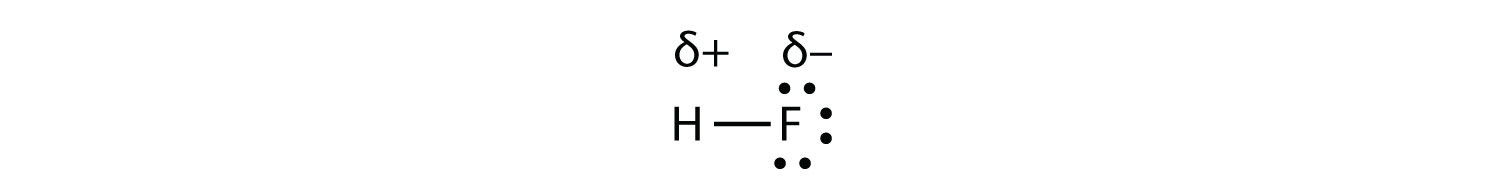
As mentioned in section 4.7, because the electrons in the bond are nearer to the F atom, this side of the molecule takes on a partial negative charge, which is represented by δ− (δ is the lowercase Greek letter delta). The other side of the molecule, the H atom, adopts a partial positive charge, which is represented by δ+. The two electrically charged regions on either end of the molecule are called poles, similar to a magnet having a north and a south pole. A molecule with two poles is called a dipole (see figure below). Hydrogen fluoride is a dipole.

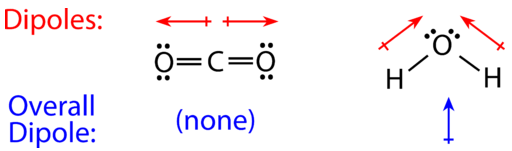
For molecules with more than two atoms, the molecular geometry must also be taken into account when determining if the molecule is polar or nonpolar. The figure below shows a comparison between carbon dioxide and water. Carbon dioxide \(\left( \ce{CO_2} \right)\) is a linear molecule. The oxygen atoms are more electronegative than the carbon atom, so there are two individual dipoles pointing outward from the \(\ce{C}\) atom to each \(\ce{O}\) atom. However, since the dipoles are of equal strength and are oriented this way, they cancel out and the overall molecular polarity of \(\ce{CO_2}\) is zero.
Water is a bent molecule because of the two lone pairs on the central oxygen atom. The individual dipoles point from the \(\ce{H}\) atoms toward the \(\ce{O}\) atom. Because of the shape, the dipoles do not cancel each other out and the water molecule is polar. In the figure below, the net dipole is shown in blue and points upward.
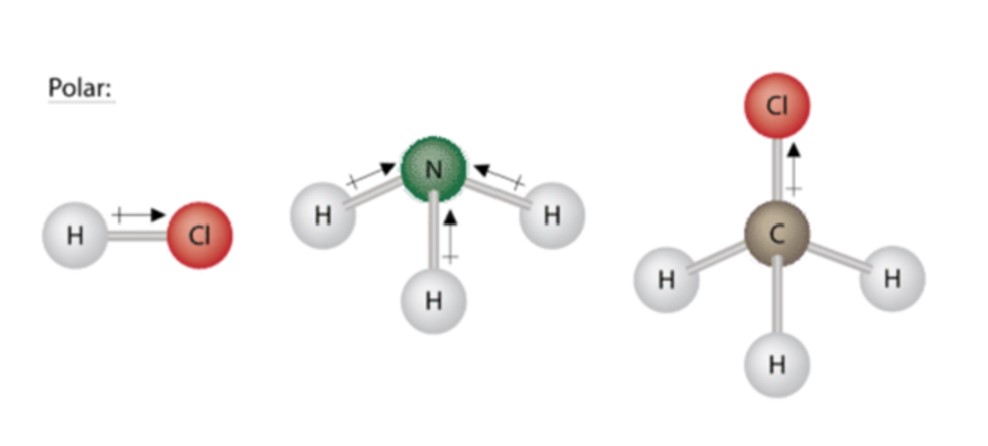
Three other polar molecules are shown below with the arrows pointing to the more electron dense atoms. Just like the water molecule, none of the bond moments cancel out.
To summarize, to be polar, a molecule must:
- Contain at least one polar covalent bond.
- Have a molecular structure such that the sum of the vectors of each bond dipole moment does not cancel.
- Draw the Lewis structure
- Figure out the geometry (using VSEPR theory)
- Visualize or draw the geometry
- Find the net dipole moment (you don't have to actually do calculations if you can visualize it)
- If the net dipole moment is zero, it is non-polar. Otherwise, it is polar.
Label each of the following as polar or nonpolar.
- Water, H2O:
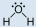
- Methanol, CH3OH:
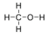
- Hydrogen Cyanide, HCN:
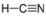
- Oxygen, O2:
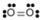
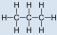
- Propane, C3H8:
Solution
- Water is polar. Any molecule with lone pairs of electrons around the central atom is polar.
- Methanol is polar. This is not a symmetric molecule. The \(\ce{-OH}\) side is different from the other 3 \(\ce{-H}\) sides.
- Hydrogen cyanide is polar. The molecule is not symmetric. The nitrogen and hydrogen have different electronegativities, creating an uneven pull on the electrons.
- Oxygen is nonpolar. The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount.
- Propane is nonpolar, because it is symmetric, with \(\ce{H}\) atoms bonded to every side around the central atoms and no unshared pairs of electrons.
Label each of the following as polar or nonpolar.
a. SO3
b. NH3
- Answer a
-
non polar
- Answer b
-
polar
Summary
- Non polar molecules are symmetric with no unshared electrons.
- Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms with different electronegativities bonded.
Source: https://chem.libretexts.org/Courses/Sacramento_City_College/SCC:_CHEM_330_-_Adventures_in_Chemistry_%28Alviar-Agnew%29/04:_Chemical_Bonds/4.12:_Shapes_and_Properties-_Polar_and_Nonpolar_Molecules
0 Response to "Easy Way to Know Polar or Nonpolar"
Post a Comment